A
Adam H
Guest
Draw Lewis structures to determine which of the following compounds exhibit resonance. Enter the letters corresponding to all those compounds which exhibit resonance.
A) ClO2
B) SF4
C) CO2
D) NO3-
E) NO2+
F) N2O4
my lewis structures for each one of these is: (can u tell me if i drew them right and which ones exhibit resonance i dont understand)
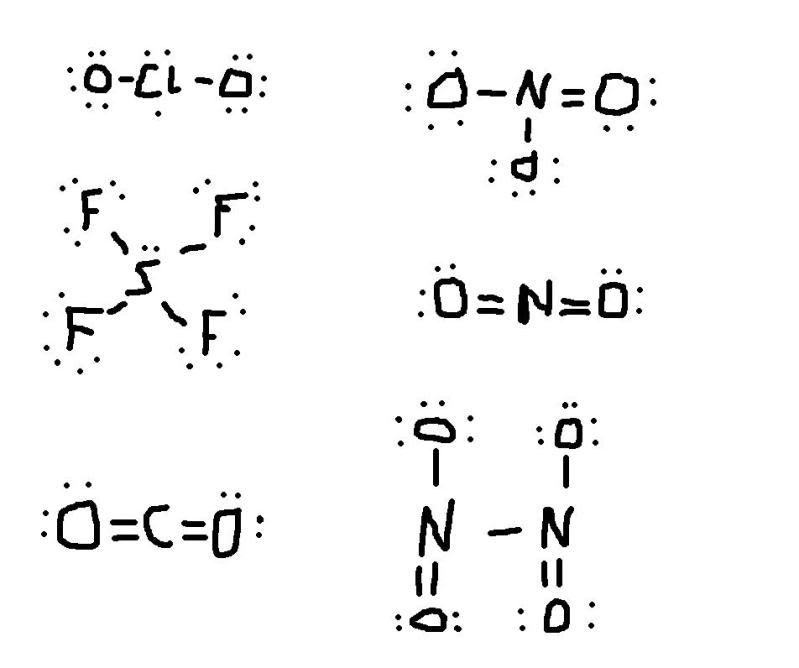
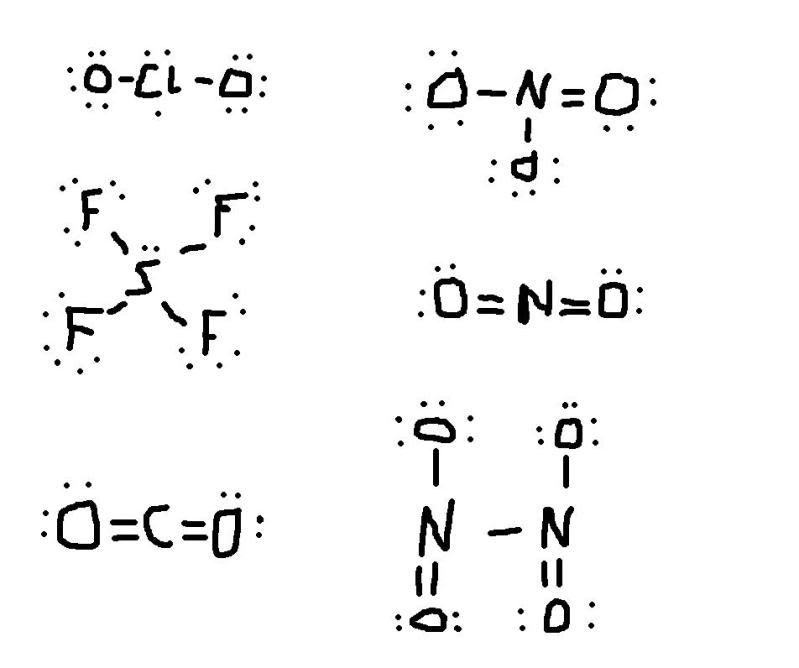
<a href="http://s80.photobucket.com/albums/j171/sykohawk/?action=view¤t=chem.jpg" target="_blank"><img src="http://i80.photobucket.com/albums/j171/sykohawk/chem.jpg" border="0" alt="Photobucket"></a>
link to see my lewis structures:
http://i80.photobucket.com/albums/j171/sykohawk/chem.jpg
A) ClO2
B) SF4
C) CO2
D) NO3-
E) NO2+
F) N2O4
my lewis structures for each one of these is: (can u tell me if i drew them right and which ones exhibit resonance i dont understand)
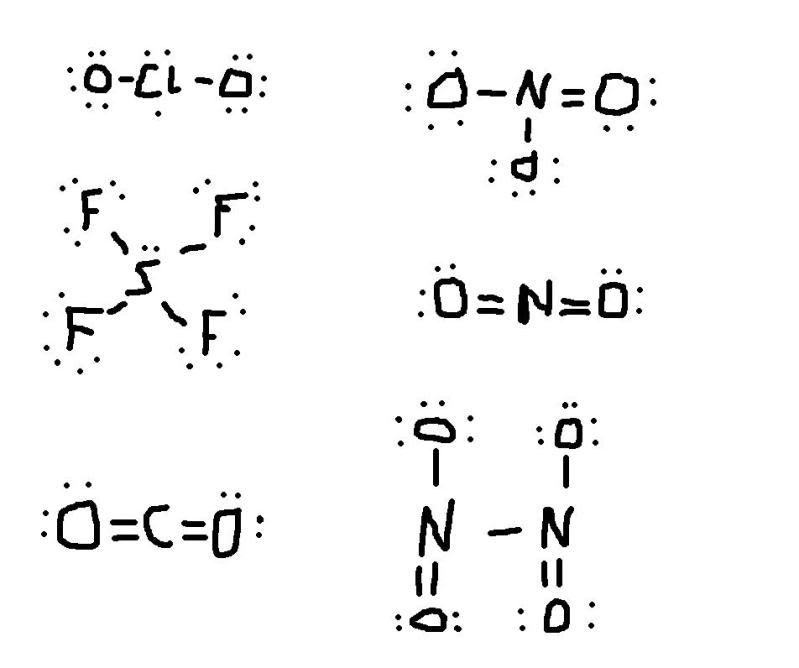
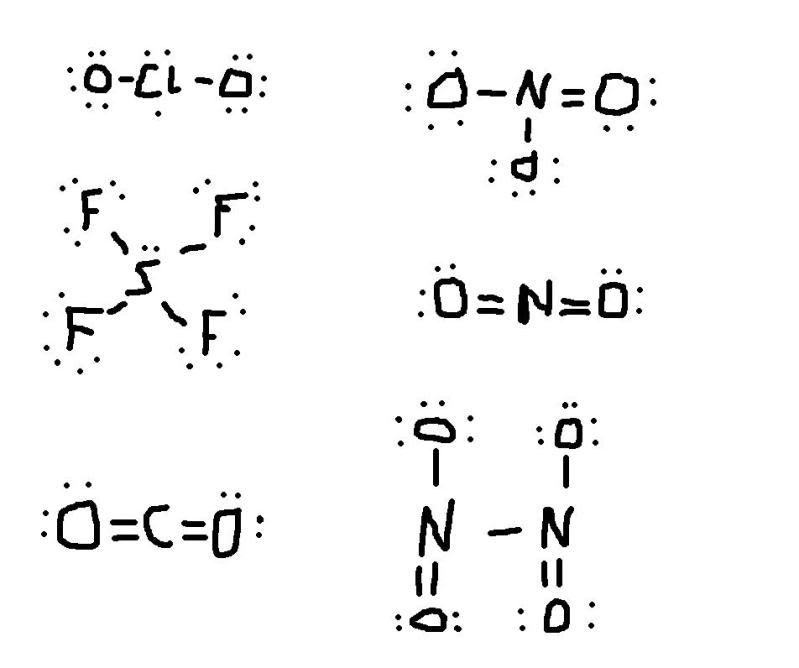
<a href="http://s80.photobucket.com/albums/j171/sykohawk/?action=view¤t=chem.jpg" target="_blank"><img src="http://i80.photobucket.com/albums/j171/sykohawk/chem.jpg" border="0" alt="Photobucket"></a>
link to see my lewis structures:
http://i80.photobucket.com/albums/j171/sykohawk/chem.jpg
